Osseointegration improvement of dental prosthetic devices through autologous growth factors permeation of the implant surface

Funding Source:
DM MiSE 01 June 2016 – Horizon 2020 – PON 2014/2020
Project code:
F/050370/01/X32
Concession decree:
578 del 02/03/2018
Start date: 02/11/2019
End date: 01/05/2022
Project Partner :
- Silfradent s.r.l.
- Università del Salento
- Andrea Francesco Palermo
Scientific manager:
Prof.ssa Luisa Siculella
Places of performance :
- Silfradent s.r.l. – Santa Sofia (FC) , via G. Di Vittorio, 35/37 – Lecce, via A. Moro, 22
- Università del Salento – Lecce, via Monteroni
- Andrea Francesco Palermo – Lecce, via A. Moro, 22
Total cost:
- € 1.847.458,67 Industrial Research (RI)
- € 439.986,88 Experimental Development (SS)
General objective:
The aim of this project is to improve the osseointegration characteristics of titanium dental implants. The research activity focuses on the adhesion of autologous growth factors, extracted with innovative techniques from the patient’s venous blood, on the surface of the device, thus obtaining a biologically active implant surface.
Objectives to be achieved:
- OR1:Design of Medical Devices for the Treatment of Autologous Growth Factors – Plant Development: Materials-Shapes-Surfaces (RI) (SILFRADENT)
- OR2: Development of experimental prototypes of medical devices – Improvement of adhesion protocols / permeation of growth factors on the plant (SS) (SILFRADENT)
- OR3: Molecular and cytological characterization of enriched fractions in growth factors and implants in in vitro systems (RI) (UNISALENTO)
- OR4: Characterization of the effects of enriched fractions in growth factors and implants on neo-vascularization capacity in in-vitro angiogenesis models (RI) (UNISALENTO)
- OR5: Preliminary clinical evaluation (SS) (STUDIO DOTT. PALERMO)
- OR6: Statistical analysis of the data (RI) (UNISALENTO)
- OR7: Research analysis and possible protocols modification (SS) (SILFRADENT)
Expected results:
One of the problems associated with titanium implants is their low wettability which requires immersion times in the biological matrix that are too long for clinical practice
The partners of this proposal intend to develop a protocol that makes possible the permeation process of the concentrate of growth factors on the titanium surface, using a dedicated implant vial, which allows the procedure to be carried out in a closed field, and a related device to the vial that allows incorporation in a few seconds.
This project aims to achieve a titanium dental implant, applicable to routine surgical practice, characterized by a biologically active surface rich in angiogenic growth factors and autologous stem cells able to improve the success of dental implant surgery and the duration of prostheses over time. To achieve this result it will be necessary to modify the implant design and the microstructure of the titanium surface to make it more available to the adhesion of the autologous biological matrix (CGF) and to reduce the time taken by the procedure with which the GFC adheres to the installation, by innovating the current process.
The achievement of this objective involves in vitro studies of the characteristics of the implant-CGF device to ensure that during the incorporation process the growth factors remain in the system and are released in situ, facilitating the healing process.
At a later stage, the validation of the device will be carried out through a clinical evaluation that involves the recruitment of volunteer subjects in which the device will be implanted with the biologically active surface realized in this project, comparing it to standard procedure. The success rate of dental implants, the duration of osteointegration times and the duration of the implants applied will be evaluated.
The repercussions of the research activity are not limited to the titanium dental implant but range in different surgical practices where there is the need to accelerate the healing processes.
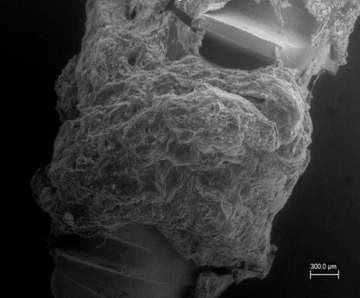
Electron microscopy image of the interface device on which the CGF fraction was deposited
Figure 9 – APAG TWIN prototype

Figure 10 – Inner view of heating elements

Figure 11 – Power card view

Figure 12 – Front view heating elements.